Periodic Table History
Periodic Table History
Periodic Table History – 440 BC
Democritus and Leucippus propose the idea of the atom, an indivisible particle that all matter is made of.
Periodic Table History – 330 BC
Aristotle proposes the four element theory: earth, air, fire & water
Periodic Table History – 360 BC
Plato coins term ‘elements’ (stoicheia)
Periodic Table History – 1605
Sir Francis Bacon published “The Proficience and Advancement of Learning” which contained a description of what would later be known as the scientific method.
1661
Robert Boyle published “The Sceptical Chymist” which was a treatise on the distinction between chemistry and alchemy. It also contained some of the earliest ideas of atoms, molecules, and chemical reaction marking the beginning of the history of modern chemistry.
Periodic Table History – 1754
Joseph Black isolated carbon dioxide, which he called “fixed air”.
1778
Antoine Lavoisier wrote the first extensive list of elements containing 33 elements & distinguished between metals and non-metals
Periodic Table History – 1766
Henry Cavendish discovered hydrogen as a colorless, odourless gas that burns and can form an explosive mixture with air.
1773–1774
Carl Wilhelm Scheele and Joseph Priestly independently isolated oxygen.
Periodic Table History – 1803
John Dalton proposed “Dalton’s Law” describing the relationship between the components in a mixture of gases.
1828
Jakob Berzelius developed a table of atomic weights & introduced letters to symbolize elements.
Periodic Table History – 1828
Johann Dobereiner who grouped together elements based on similarities and patterns.
1864
John Newlands arranged the known elements in order of atomic weights & observed similarities between some elements.
Periodic Table History – 1864
Lothar Meyer develops an early version of the periodic table, with 28 elements organized by valence.
History – 1864
Dmitri Mendeleev produced a table based on atomic weights but arranged ‘periodically’ with elements with similar properties under each other. His Periodic Table included the 66 known elements organized by atomic weights.
1894
William Ramsay discovered the Noble Gases.
1898
Marie and Pierre Curie isolated radium and polonium from pitchblende.
1900
Ernest Rutherford discovered the source of radioactivity as decaying atoms.
History – 1913
Henry Moseley determined the atomic number of each of the elements and modified the ‘Periodic Law’.
History – 1940
Edwin McMillan and Philip H. Abelson identify neptunium, the lightest and first synthesized transuranium element, found in the products of uranium fission.
History – 1940
Glenn Seaborg synthesised transuranic elements (the elements after uranium in the periodic table).
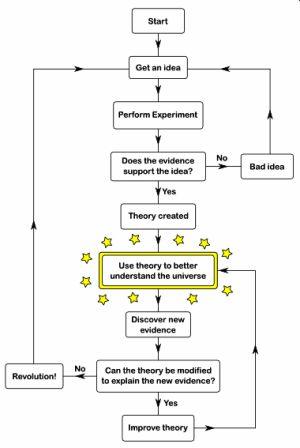
Periodic Table History – The Theory of the Periodic Table
All of the above scientists contributed to the history of the Periodic Table and Periodic Chemistry. The following flowchart illustrates the process of their work, as it does for all aspects of developing a scientific theory.